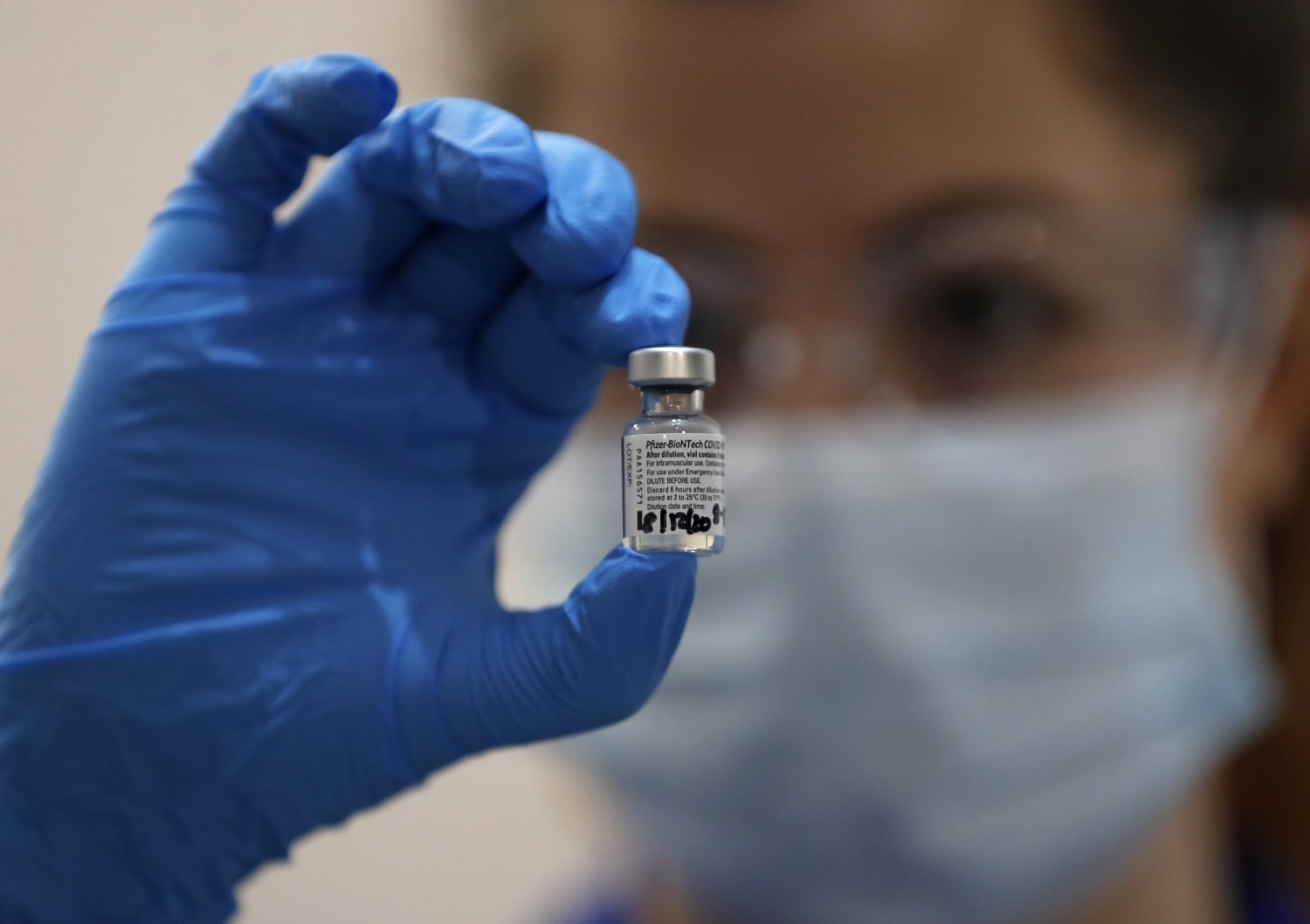
On Thursday, the Food and Drug Administration’s regulatory vaccines committee voted to recommend the COVID-19 vaccine developed by Pfizer and BioNTech for emergency use authorization.
The vote by the FDA Vaccines and Related Biological Products Advisory Committee doesn’t mean the vaccine gets immediate approval by the FDA. That decision will be made at a future time that has yet to be announced. A CDC advisory committee is scheduled to meet Sunday.
The vote concluded after a meeting in which more than a dozen experts discussed the results of Pfizer’s advance trial. Emergency use authorization is the final step necessary before millions of doses can be shipped to the American public. The Pfizer-BioNTech coronavirus vaccine has already been approved for emergency use in Britain and Canada.
Editorial credit: Mike Mareen / Shutterstock.com